Vapours condense when they become cool enough. This process is based on the principle that different substances boil at different temperatures.

Which Are The Products Of Fractional Distillation Of Crude Oil Quora
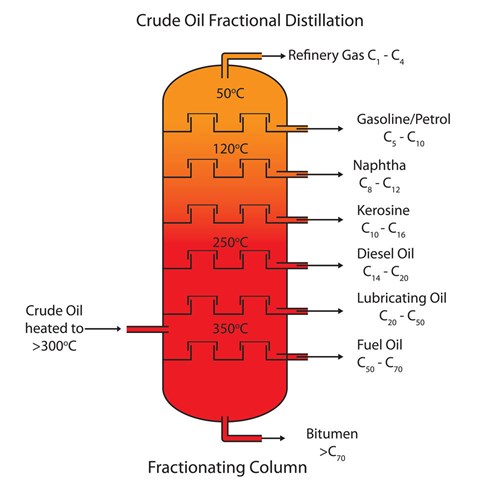
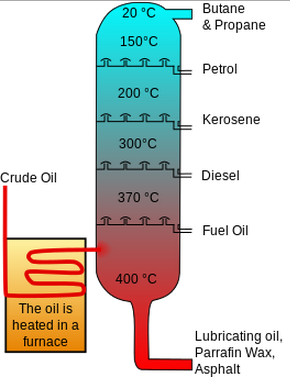
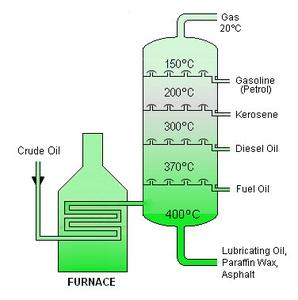
Heated crude oil enters a tall fractionating column which is hot at the bottom and gets cooler towards the top.

. This involves heating crude oil to about 350 degrees Celsius to turn it into a mixture of gases. The Petroleum is heated up and the gas produced. Refinery Gases bottled gas heating Gasoline fuel for cars Naphtha starting material to make.
Vapours from the oil rise through the column. Answer 1 of 3. Different fractions condense at certain temperature ranges.
Learn vocabulary terms and more with flashcards games and other study tools. Fractional distillation is the process by which oil refineries separate crude oil into different more useful hydrocarbon products based on their relative molecular weights in a distillation tower. The crude oil is heated to about 600 C and its vapours are allowed to condense at different temperatures in the fractionating.
A tall fractionating column is fitted above the mixture with. The substances in crude oil have different boiling points so they can be separated by fractional distillation. New feed is always being added to the distillation column and products are always being removed.
Fractional distillation separates a mixture into a number of different parts called fractions. On the right you can see several chemical processors that are described in the next section. This is the first step in the processing of crude oil and it is considered to be the main separation process as it performs the initial rough separation of the different fuels.
A common example of fractional distillation in industries is the separation of various components of crude oil. The process of separating the various components of petroleum from one another is known as the refining of petroleum. Crude oil is added in the chamber and is heated with high-pressure.
Summarized form of different fractions obtained after Fractional distillation of Petroleum. In most cases the distillation is operated at a continuous steady state. Fractional distillation of crude oil.
The first thing that happens to crude oil is that it is fractionally distilled though trendy technology has precipitated a shift to chemical distillation by using excessive-tech chemical processes and reactions to separate the completely different grades of hydrocarbons though in some cases they need to combine hydrocarbons a process often called unification. Crude oil normally contains substances such as paraffin wax gasoline diesel naphtha lubricating oil and kerosene. On a very basic level the fractional distillation works because different fractions of crude oil have different boiling points - temperature at which they start to evaporate.
The process of separating a mixture into a series of fractions of different volatilities by means of distillation is known as fractional distillation. The fractions are separated from one another using a process called fractional distillation. Vapours from the oil rise through the column.
On an industrial scale the different fractions of Petroleum are separated out by fractional distillation. Fractional distillation heated crude oil enters a tall fractionating column which is hot at the bottom and gets cooler towards the top. Petrol used as a fuel.
Petroleum can be divided into products such as petrol diesel kerosene naptha etc. The process involved here is fractional distillation in. The process through which petroleum is refined is called fractional distillation and occurs in a distillation tower or fractionating column.
Very few of the components come out of the fractional. Fractional distillation is a process of separating the compounds of the mixture on the basis of differences in their boiling points. Fractional distillation is the most common form of separation technology used in petroleum refineries petrochemical and chemical plants natural gas processing and cryogenic air separation plants.
The chemicals in a certain fraction are hydrocarbons with comparable numbers of carbon atoms. Crude oil separated into fractions containing a mixture of hydrocarbons with similar boiling points. Fractional distillation is the use of distillation to separate a liquid mixture into different parts fractions that differ in boiling point.
Petroleum can be refined by fractional distillation since it separates crude petroleum into useful fractions such as gasoline kerosene oil lubricating oil etc. This is done by a process called fractional distillation which is based on the fact that the different components of petroleum have. 256 describe and explain the separation of crude oil by fractional distillation.
This principle allows the evaporating fraction to be directed to its. Refinery gases used for bottled gases. Gasoline and many other chemicals are produced from crude oil using fractional distillation.
The oil refining process starts with a fractional distillation column. Crude Oil. For crude oil to be used effectively by modern industry it has to be separated into its component parts and have impurities like sulfur removed.
Vapours condense when they become cool enough. Outside of the oil industry fractional distillation is a process used for separating a mixture of two or more fluids of different densities by heating the mixture to the point of boiling. For example crude oil contains kerosene and naphtha which are useful fractions naphtha.
Petroleum is refined by fractional distillation. Liquids are led out of the column at different heights. Lubricating oils and greases.
Petroleum can be separated into various types of fuel by a process called refining using fractional distillation. Start studying Crude Oil and fractional distillation. Asphalt or petroleum coke.
The different hydrocarbon components of crude oil are called fractions. 257 describe the fractions as largely a mixture of compounds of formula CₙH₂ₙ₂ which are members of the alkane homologous series and recall the names and uses of the following fractions. The most common method of refining crude is the process of fractional distillation.
In the process of fractional distillation a mixture of different liquids is evaporated followed by condensation. The distillation process helps in separating these components effectively. Sorry for bad spelling i think this is right.
Fractional distillation is useful for separating a mixture of substances with narrow differences in boiling points and is the most important step in the refining process. Crude oil is heated until it evaporates. Petrol Diesel and Kerosene are all products or fractions of the process of refining Petroleum.
How To Demonstrate Fractional Distillation In A Science Class Philip Harris

Fractional Distillation Of Crude Oil Refining Petroleum Products Crown Oil
0 Comments